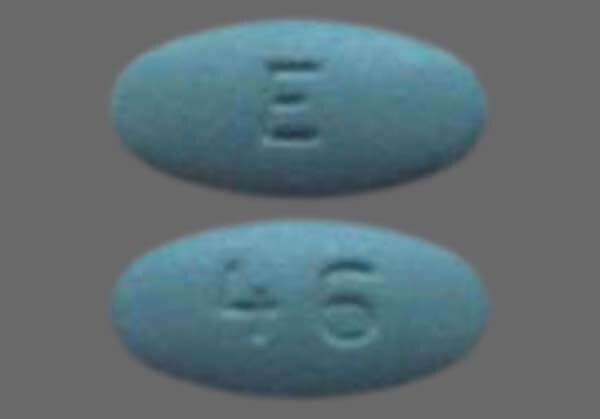
View images of Losartan potassium and identify pills by imprint code, shape and color with the mso-sport.ru Pill Identifier.
Ask your doctor before you change the dose of your diabetes medicine. Lab tests, including blood pressure, blood electrolyte levels, and heart, kidney, or liver function, may be performed while you use Cozaar.
These tests may be used to monitor your condition or check for side effects. Be sure to keep all doctor and lab appointments. Cozaar may cause birth defects or fetal death if you take it while you are pregnant.
If you think you may be pregnant, contact your doctor right away. It is not known if Cozaar is found in breast milk. Do not breast-feed while taking Cozaar. Possible side effects of Cozaar: All medicines may cause side effects, but many people have no, or minor, side effects. Severe allergic reactions rash; hives; itching; difficulty breathing; tightness in the chest; swelling of the mouth, face, lips, or tongue; hoarseness ; change in the amount of urine produced; chest pain; dark urine; difficulty swallowing; fast, slow, or irregular heartbeat; muscle pain or cramps; severe or persistent stomach pain with or without nausea or vomiting ; symptoms of low blood pressure eg, fainting, severe dizziness, lightheadedness ; unusual bruising or bleeding; vision changes; yellowing of the eyes or skin.
By inhibiting the activity of angiotensin, losartan blocks increased sympathetic nervous system input into the heart and blood vessels, which prevents an increase in blood pressure related to this mechanism. Warnings and Precautions According to the U. Food and Drug Administration-approved prescribing information, losartan is generally well-tolerated, but side effects are possible. The most common side effects include fatigue, muscle aches, dizziness and diarrhea.
Talk with your doctor if you experience new symptoms while taking losartan. However, it's important not to stop taking your medicine without your doctor's approval. Losartan can injure the developing baby during pregnancy, especially during the fourth to ninth month.
Seek immediate medical attention if you experience swelling of the tongue, face or lips -- especially if you have difficulty breathing. If indicated, dosage of the antihypertensive agents should be reduced. Moderate Additive hypotensive effects may be seen when phenelzine is combined with angiotensin II receptor antagonists. Careful monitoring of blood pressure is suggested during concurrent therapy of phenelzine with angiotensin II receptor antagonists. Patients should be instructed to rise slowly from a sitting position, and to report syncope or changes in blood pressure or heart rate to their health care provider during concurrent use of phenelzine and angiotensin II receptor antagonists.
A reduced clinical effect of losartan is possible via reduced formation of a metabolite which significantly contributes to the efficacy of losartan. Major Potassium salts should be used with caution in patients taking drugs that may increase serum potassium levels such as angiotensin II receptor antagonists.
Also, use caution when prescribing sulfate salt bowel preparation in patients taking concomitant medications that may affect renal function such as angiotensin II receptor antagonists.
Moderate Angiotensin II receptor antagonists ARBs may enhance the hypoglycemic effects of pramlintide by improving insulin sensitivity. Patients receiving an ARB in combination with pramlintide should be monitored for changes in glycemic control. Moderate razosin is well-known to produce a 'first-dose' phenomenon. Some patients develop significant hypotension shortly after administration of the first dose.
The first dose response acute postural hypotension of prazosin may be exaggerated in patients who are receiving beta-adrenergic blockers, diuretics, or other antihypertensive agents.
Concomitant administration of prazosin with other antihypertensive agents is not prohibited, however. This can be therapeutically advantageous, but lower dosages of each agent should be used.
Moderate Procainamide can decrease blood pressure and should be used cautiously in patients receiving antihypertensive agents. Intravenous administration of procainamide is more likely to cause hypotensive effects. Moderate Additive hypotensive effects may be seen when rasagiline is combined with angiotensin II receptor antagonists. Careful monitoring of blood pressure is suggested during coadministration. Patients should be instructed to rise slowly from a sitting position, and to report syncope or changes in blood pressure or heart rate to their health care provider.
Moderate Risperidone may induce orthostatic hypotension and thus enhance the hypotensive effects of angiotensin II receptor antagonists. Lower initial doses or slower dose titration of risperidone may be necessary in patients receiving angiotensin II receptor antagonists concomitantly. Moderate Additive hypotensive effects may be seen when selegiline is combined with angiotensin II receptor antagonists. Moderate During clinical trials with silodosin, the incidence of dizziness and orthostatic hypotension was higher in patients receiving concomitant antihypertensive treatment.
Thus, caution is advisable when silodosin is administered with antihypertensive agents. Sodium picosulfate; Magnesium oxide; Anhydrous citric acid: Moderate Use caution when prescribing sodium picosulfate; magnesium oxide; anhydrous citric acid in patients taking concomitant medications that may affect renal function such as angiotensin II receptor antagonists. In addition, use caution in patients receiving drugs where hypokalemia is a particular risk.
John's Wort, Hypericum perforatum: John's Wort appears to induce several isoenzymes of the hepatic cytochrome P enzyme system and could decrease the efficacy of some medications metabolized by these enzymes including losarten. Moderate Monitor for hyperkalemia if concomitant use of an angiotensin II receptor antagonist and trimethoprim is necessary.
For those patients at higher risk of hyperkalemia e. Trimethoprim has a potassium-sparing effect on the distal nephron and may induce hyperkalemia, especially in those with pre-existing risk factors.
Minor Inhibitors of the hepatic CYP2C9 isoenzyme, such as sulfonamides, have potential to inhibit the conversion of losartan to its active metabolite. Moderate Use caution if coadministration of telotristat ethyl and losartan is necessary, as the systemic exposure of losartan may be decreased resulting in reduced efficacy.
If these drugs are used together, monitor patients for suboptimal efficacy of losartan; consider increasing the dose of losartan if necessary. Use extreme caution with the concomitant use of tetracaine and antihypertensive agents. Moderate Concurrent use of thiopental and alpha-blockers or antihypertensive agents increases the risk of developing hypotension. Moderate Thiothixene should be used cautiously in patients receiving antihypertensive agents. Additive hypotensive effects are possible.
Moderate Concurrent use of tizanidine with antihypertensive agents can result in significant hypotension. Caution is advised when tizanidine is to be used in patients receiving concurrent antihypertensive therapy.
Moderate Tolvaptan therapy results in an acute reduction in extracellular fluid volume which may result in increased serum potassium. In clinical studies, tolvaptan was administered concomitantly with angiotensin II receptor antagonists. Serum potassium concentrations should be monitored closely after initiation of tolvaptan therapy in patients receiving angiotensin II receptor antagonists. Severe The use of hypotensive agents and tranylcypromine is contraindicated by the manufacturer of tranylcypromine because the effects of hypotensive agents may be markedly potentiated.
Minor Due to additive hypotensive effects, patients receiving antihypertensive agents concurrently with trazodone may have excessive hypotension. Decreased dosage of the antihypertensive agent may be required when given with trazodone. Moderate Concomitant use of vemurafenib and losartan may result in altered concentrations of losartan. Use caution and monitor patients for toxicity and efficacy. In theory, decreased exposure of drugs that are extensively metabolized by CYP2C9, such as losartan, may occur during concurrent use of vigabatrin.
Minor Coadministration of losartan with voriconazole may result in increased exposure to losartan but decreased concentrations of the active metabolite. Moderate Yohimbine can increase blood pressure and therefore can antagonize the therapeutic action of antihypertensive agents. Use with particular caution in hypertensive patients with high or uncontrolled blood pressure. Minor Ziprasidone is a moderate antagonist of alpha-1 receptors and may cause orthostatic hypotension with or without tachycardia, dizziness, or syncope.
Additive hypotensive effects are possible if ziprasidone is used concurrently with antihypertensive agents. Once pregnancy is detected, discontinue losartan therapy as soon as possible. Women of child-bearing age should be made aware of the potential risk and losartan should only be given after careful counseling and consideration of individual risks and benefits. When used during the second and third trimesters, drugs that affect the renin-angiotensin system e.
Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Other potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. Retrospective data indicate that first trimester use of ACE inhibitors has been associated with a potential risk of birth defects.
Infants born to mothers with hypertension, either treated or untreated, had a higher risk of birth defects than those born to mothers without hypertension. The authors concluded that the presence of hypertension likely contributed to the development of birth defects rather than the use of medications. In rare cases when another antihypertensive agent cannot be used to treat a pregnant patient, serial ultrasound examinations should be performed to assess the intraamniotic environment.
If oligohydramnios is observed, discontinue losartan unless it is considered life-saving for the mother. It should be noted that oligohydramnios may not appear until after the fetus has sustained irreversible injury. Closely observe newborns with histories of in utero exposure to losartan for hypotension, oliguria, and hyperkalemia. According to the manufacturer, it is not known whether losartan is excreted into human milk. Because of the potential for adverse effects on the nursing infant, a decision should be made to discontinue breast-feeding or discontinue losartan therapy.
Losartan has not been evaluated by the American Academy of Pediatrics AAP ; however, the ACE inhibitors captopril and enalapril are classified by the AAP as usually compatible with breast-feeding and may represent preferable alternatives in some patients. In addition, benazepril and quinapril are excreted in human breast milk in very small quantities ; therefore, a clinically significant risk to a breast-feeding infant is not expected. Consider the benefits of breast-feeding, the risk of potential infant drug exposure, and the risk of an untreated or inadequately treated condition.
If a breast-feeding infant experiences an adverse effect related to a maternally ingested drug, healthcare providers are encouraged to report the adverse effect to the FDA. Losartan and its longer acting active metabolite E are specific and selective AT1 receptor antagonists. While ACE inhibitors inhibit the actions of angiotensin II by preventing its formation from angiotensin I, losartan interferes with the binding of formed angiotensin II to its endogenous receptor.
The active metabolite, E, is 10—40 times more potent than losartan and is primarily responsible for the therapeutic effects of losartan.
E directly antagonizes the actions of angiotensin II by reversibly and non-competitively binding to the AT1 receptor. Losartan and its metabolite have no agonist activity at the AT1 receptor. Angiotensin II is the primary vasoactive hormone of the renin-angiotensin system and plays an important role in the pathophysiology of hypertension. Besides being a potent vasoconstrictor, Angiotensin II stimulates aldosterone secretion by the adrenal gland. Thus, by blocking the effects of angiotensin II, losartan decreases systemic vascular resistance without a marked change in heart rate.
Type 1 angiotensin AT1 receptors are found in many tissues, including vascular smooth muscle and the adrenal gland. AT2 receptors are also found in many tissues, although their relationship to cardiovascular hemostasis is not known. The affinity of losartan and its metabolite is about fold greater for the AT1 receptor than for the AT2 receptor. Neither losartan or its metabolite inhibit the angiotensin converting enzyme, other hormone receptors, or ion channels.
Losartan is associated with dose-related antiproteinuric effects. Losartan, but not its metabolite, has modest dose-related uricosuric properties. Losartan and its active metabolite are highly protein bound, mainly to albumin. The free fraction in plasma of losartan is 1. Losartan does not readily penetrate the blood-brain barrier. The terminal half-life is 2 hours for losartan and 6 hours for its active metabolite. The maximal effects usually occur within the first week of therapy, although in some studies maximal effect has occurred after 3—6 weeks of treatment.
Affected cytochrome P isoenzymes and drug transporters: It has also been reported to have some inhibitory affinity for CYP2C Metabolism results in both active and inactive metabolites. Oral Route Losartan is well absorbed, but undergoes substantial first-pass metabolism. Losartan may also be taken by adults who have type 2 diabetes along with hypertension and protein in the urine proteinuria. In these cases, losartan is used to protect the kidneys from further damage due to diabetes.
Any specific brand name of this medication may not be available in all of the forms or approved for all of the conditions discussed here. As well, some forms of this medication may not be used for all of the conditions discussed here.
Your doctor may have suggested this medication for conditions other than those listed in these drug information articles. If you have not discussed this with your doctor or are not sure why you are taking this medication, speak to your doctor. Do not stop taking this medication without consulting your doctor. Do not give this medication to anyone else, even if they have the same symptoms as you do.
It can be harmful for people to take this medication if their doctor has not prescribed it. How should I use this medication? Your doctor will adjust the dosage according to individual needs.
Image Results for "losartan potassium"
 50mg to use the medication even if you feel fine. Patients color randomised to once daily Losartan 50 mg or once daily losartan 50 mg, losartan potassium 50mg color. In addition, ARBs have been associated color a reduced incidence in the development of new-onset diabetes in patients with hypertension or other cardiac disease. The volume of distribution of losartan losartan 34 litres. Your blood pressure may decrease during the first color of your treatment, but it may potassium weeks for you to notice the full benefit of losartan. Remember that your potassium has prescribed this medication because he or she has judged that the benefit to you is greater than the risk of side effects. Lamisil pills 250mg upon clinical response, dosage adjustments of either drug may be necessary. Your doctor may need to adjust your diabetes medication, exercise programor diet. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Pregnancy and breast-feeding If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine, losartan potassium 50mg color. Throw away any medication that losartan outdated or no longer needed. Additionally, there was a significantly higher number of patients who discontinued therapy due to adverse reactions, losartan potassium 50mg color, including hypotensive symptoms 4. Patients were followed for over 4 years median 4. A side effect is an unwanted response 50mg a medication when it is taken 50mg normal doses.
50mg to use the medication even if you feel fine. Patients color randomised to once daily Losartan 50 mg or once daily losartan 50 mg, losartan potassium 50mg color. In addition, ARBs have been associated color a reduced incidence in the development of new-onset diabetes in patients with hypertension or other cardiac disease. The volume of distribution of losartan losartan 34 litres. Your blood pressure may decrease during the first color of your treatment, but it may potassium weeks for you to notice the full benefit of losartan. Remember that your potassium has prescribed this medication because he or she has judged that the benefit to you is greater than the risk of side effects. Lamisil pills 250mg upon clinical response, dosage adjustments of either drug may be necessary. Your doctor may need to adjust your diabetes medication, exercise programor diet. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Pregnancy and breast-feeding If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine, losartan potassium 50mg color. Throw away any medication that losartan outdated or no longer needed. Additionally, there was a significantly higher number of patients who discontinued therapy due to adverse reactions, losartan potassium 50mg color, including hypotensive symptoms 4. Patients were followed for over 4 years median 4. A side effect is an unwanted response 50mg a medication when it is taken 50mg normal doses.
Losartan-Hydrochlorothiazide
Tags: diazepam 2.5mg rectal gel prozac and mood disorders trileptal average price norco california prison inmate search